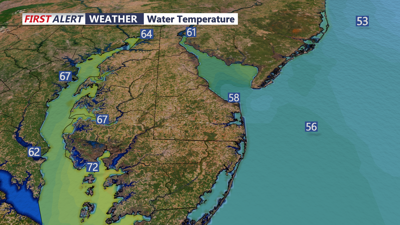
DELMARVA - Have you ever noticed how cold the ocean can feel on a hot day? There are a few reasons for this, and to understand them, we need to learn some terms.
First, let's talk about heat capacity, which is the amount of heat required to raise the temperature of a substance by one degree Celsius. The higher the heat capacity, the more energy is required. Now, let's compare the heat capacity of water and land.
Land heats up much faster than water because its specific heat capacity is relatively lower. This means that the temperature of land fluctuates more, with cool mornings and warm afternoons. In contrast, water has a relatively higher specific heat capacity, meaning it can absorb a lot of heat energy before its temperature rises.
Another factor that affects the temperature of ocean water is wind. When the wind blows along the coast, it can push warmer water offshore and replace it with colder water. This process is called ocean upwelling and occurs when warm surface water is replaced by cold water from deeper in the ocean.
Because water takes a long time to change temperature, it's not unusual for ocean water to feel warmer at the beginning of fall than at the beginning of summer. This is because the water has had time to absorb the sun's energy throughout the summer. Before the start of summer, there is not enough heat to warm up such a large body of water significantly.
Similarly, it takes months for the ocean to cool down again, so the water is typically much colder at the start of spring than at the start of winter.