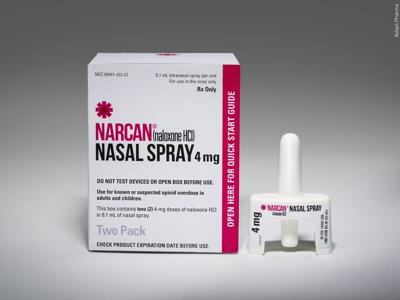
GAITHERSBURG, Md. - The U.S. Food and Drug Administration has accepted an application from Emergent BioSolutions Inc. for NARCAN Nasal Spray as an over-the-counter emergency treatment for known or suspected opioid overdoses.
If approved, NARCAN would be the first 4mg naloxone nasal spray available over the counter in America. The application was granted priority review by the FDA.
According to Emergent's website, their submission to the FDA includes conducted human factors studies and more than five years of post-marketing data to demonstrate the safe and effective use of NARCAN. Emergent has distributed prescription NARCAN devices to health departments and first responders closest to at-risk populations since its approval in 2015.
This application comes at the same time as a report from the Centers for Disease Control and Prevention, which analyzed overdose deaths among people between the ages of 10-19 in recent years. This new report says that overdoses among people in this age group increased 109% from the second half of 2019 to the second half of 2021, with nearly 90% of deaths involving opioids. Deaths involving illicitly manufactured fentanyls increased 182%.
Multiple Delaware agencies have shared concerns regarding the use of fentanyl by youth in the state. Last month the Delaware Division of Public Health announced a campaign to warn people of the dangers of fentanyl and highlight the risk of experimental drug use and the evolving drug market.
More information regarding naloxone, including how to have it mailed to your house and training programs, can be found at delaware.gov and helpisherede.com.